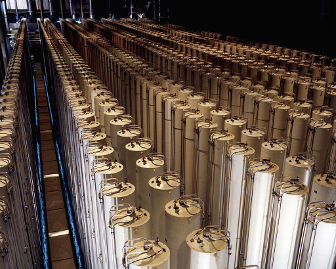
Uranium enrichment gas centrifuge cascade in Piketon, Ohio plant in 1984. Public domain photo from the U.S. Department of Energy.
by John Walker

|
|
Uranium enrichment gas centrifuge cascade in Piketon, Ohio plant in 1984. Public domain photo from the U.S. Department of Energy. |
We may be entering a new era of proliferation of nuclear weapons. During the Cold War, a largely bipolar world was dominated by extremely large U.S. and Soviet nuclear arsenals, with smaller acknowledged nuclear forces maintained by Britain, France, and China. Israel was believed to have nuclear weapons but never declared itself a nuclear weapons state. India exploded a nuclear device in 1974 but claimed it was a test for peaceful purposes and denied any plans to build a nuclear stockpile. South Africa was rumoured to have developed nuclear weapons and in 1993 confirmed that it had developed weapons which subsequently were dismantled. In 1998 India officially joined the nuclear weapons club by testing five devices, including one which was claimed to be a thermonuclear weapon. Pakistan rapidly responded with their own nuclear tests in 1998. In 2006 North Korea conducted an underground test of a low yield device (so low in yield it may have been a fizzle) and followed up with tests in 2009 and 2013.
Libya, Iraq, Syria, and Iran have pursued nuclear weapons programs. In the aftermath of the invasion of Iraq in 2003, countries in the region had to weigh the risk of being invaded if they were believed to be developing nuclear weapons against the deterrence to invasion that being known (or believed) to possess a nuclear weapon might confer. The experience of North Korea demonstrates to any rogue state that possession of just a few nuclear weapons shields one against foreign intervention and can be used to extract concessions in negotiations. There is thus an incentive for dodgy regimes to build these weapons, which is amplified when other rival dodgy regimes on their borders are seen or suspected of also building them. There are essentially no secrets remaining in the construction of simple fission weapons (building thermonuclear [fusion] weapons is much more difficult, but in any case mastering fission technology is a prerequisite to building a fusion weapon). Given the fissile material (uranium or plutonium), just about everything you need to know about how to build a simple fission bomb is available at the library or on the Internet. Attempts to restrict the proliferation of nuclear weapons, then, concentrate upon restricting access to fissile material.
All deployed nuclear weapons use one of two fissile isotopes: Uranium-235 (U-235) or Plutonium-239 (Pu-239). In this essay we'll examine the process by which U-235 is extracted from natural uranium. In a companion essay the process of producing plutonium from uranium and separating it for use in weapons will be examined.
As negotiations proceed with potential nuclear weapon states, a central issue is the enrichment of natural uranium to produce U-235. Details matter, and often media coverage glosses over technical matters upon which the controversy hinges. It's important to grasp the fundamentals of uranium enrichment and its consequences for the ability to build a nuclear weapon. So let's dive into the gnarly specifics. I will concentrate on the technologies most commonly used today and leave out many details. Think of this as an executive briefing on what you need to know about any proposed agreement. If you follow the links in the material that follows, you will find more information on these topics.
Natural
uranium, extracted from uranium ore, is composed primarily of
two isotopes (isotopes
are nuclei with the same number of protons but different
numbers of neutrons). Isotopes have identical chemical properties,
but can behave differently in nuclear reactions. In natural uranium,
99.284% is the heavier U-238 nucleus, while 0.711% is U-235. U-235
is the only isotope which can fission with
thermal neutrons,
which are the means of fission in nuclear
power reactors and nuclear fission weapons. It is possible to build
a power reactor which uses natural uranium, but only with a
moderator of
heavy water or
graphite.
Few modern civil nuclear power reactors use such designs,
which are expensive and inefficient. It is impossible to build a
nuclear weapon of any kind from natural uranium.
The most common design for nuclear power reactors is the light water reactor, which uses ordinary water as coolant and neutron moderator. These reactors cannot use natural uranium as fuel—they require a higher fraction of U-235 in their fuel in order to sustain a chain reaction. Light water power reactors typically use fuel enriched to between 3 and 5% U-235 (usually toward the low end of this range). These reactors would not benefit in any way from fuel enriched to a higher fraction of U-235. Keep that last detail in mind.
So, how do we go about enriching the U-235 content from the 0.7% in natural uranium to, say 3–4% for reactor fuel (call it around a factor of five). Well, it's neither easy, nor simple, nor cheap. Recall that there is no chemical difference between two isotopes of the same chemical element. This means that the only way they can be separated is by discriminating them by mass (or weight, if you like). There are a number of ways of doing this, but the most widely-used and efficient mechanism at present is the gas centrifuge. Uranium metal is reacted with fluorine to produce uranium hexafluoride gas (which is simultaneously radioactive, toxic, and corrosive—trifecta!), which is then spun in a column at very high speed. The slightly heavier molecules, containing U-238, migrate to the outside of the spinning centrifuge, while the lighter ones, with U-235, are concentrated nearer the centre. Gas, containing a slightly higher fraction of U-235, is extracted from the centre and sent on to the next stage, and gas with slightly more U-238 from near the wall of the centrifuge is fed back to the earlier stage. Now, the difference in mass between U-235 and U-238 is just 1.26% and each centrifuge contributes only a small increase in enrichment, so in practice you need a cascade of thousands of centrifuges to enrich by the desired factor of five. But note that the cascade is not a linear array. At each stage, more lower-enriched material is fed back back to the earlier stage, while a smaller fraction of higher-enriched uranium goes on to the next. Thus, the enrichment cascade is more like a pyramid than an assembly line. Please also keep this in mind.
So, after your uranium hexafluoride emerges from the cascade of thousands of centrifuges, it's enriched to a U-235 content of 3–4% (“reactor grade”) and all that remains is to convert it to uranium metal or uranium oxide and fabricate it into fuel elements for power reactors. If all you're interested in is a domestic civil nuclear power program, you're done. In fact, a substantial majority of countries with civil nuclear power stations do not have any domestic enrichment facilities, but buy enriched fuel from other countries. In any case, even if a country enriches all or part of its nuclear fuel requirements, it can easily comply with International Atomic Energy Agency (IAEA) safeguards and inspections to verify that no enrichment beyond reactor-grade is being done, and that no enriched uranium is being diverted to a covert weapons program. As of mid-2013, 181 states had active agreements with the IAEA safeguards in effect.
Now, let's suppose your nuclear ambitions go beyond civil nuclear power plants. Recall, each pass through a cascade of centrifuges enriches around 5 to 7 times—let's call it 5. Suppose you started with 5 tonnes (metric ton: 1000 kilograms; that's how people speak of nuclear material) of natural uranium and you've enriched it to 1 tonne of reactor-grade fuel. Now, instead of fabricating fuel rods, you divert it and send it back through the cascade (or into another one) for a second pass. The first thing to notice is that you only have one fifth of the input to the cascade you had on the first pass, so the mass flow is much less, and hence you need fewer centrifuges (the “width” of the cascade at each stage). The output of this enrichment will be around 20% U-235.
We started with 5 tonnes of natural uranium and ended up, after the first pass through the cascade, with one tonne of reactor-grade fuel. After the second pass, with one tonne in, we get 200 kilograms of 20% enriched uranium.
There are only two non-weapons applications for highly enriched uranium: naval propulsion, and research and medical isotope production reactors. If a country has no nuclear powered ships and does not desire to acquire nuclear weapons, there is no plausible reason to have domestic enrichment beyond 5% reactor grade. Fuel for medical isotope reactors can be obtained from abroad under IAEA safeguards, and most such reactors today have been modified to use fuel enriched to less than 20%. The fuel demand for such research reactors is minuscule compared to nuclear power stations, so there is no reason whatsoever to produce such fuel domestically when it can be purchased from other countries under IAEA safeguards.
If you don't pay attention to the size of the cascade and the amount of mass flowing through it, it's easy to confuse the degree of enrichment with the amount of work required to do it. In terms of the number of centrifuges and the energy to run them, it took five times as much to get from natural uranium to reactor fuel as it did to get from that to 20%. Now suppose you're a bad boy. Run that 200 kilograms of 20% enriched uranium through a cascade which is five times smaller than the step which produced it and 25 times smaller than the one which processed the original natural uranium (and hence correspondingly easier to conceal), and you end up with 40 kilograms or so of weapons-grade uranium (usually defined as 80% enrichment or above, although modern weapon designs usually employ uranium enriched to 90% U-235 or higher). From your 200 kilograms of 20% enriched uranium, you'll get around 40 kg of weapons grade material, which, depending upon bomb design, suffices to make a blinding flash and stunning report.
Here is the take away from these details. States suspected of operating covert nuclear weapons programs have claimed on multiple occasions that their interest is purely in a civil nuclear power program. Fine—I applaud that. Thirty-seven percent of the electrons which keep the lights on here at Fourmilab are generated by nuclear power, and I would not presume to deny that boon to any other nation. It isn't clear why such states need to have their own domestic enrichment program when other countries such as Argentina and Spain buy their nuclear fuel from abroad, but again, if they want to make the investment, it's up to them. But any state which is a signatory to the Treaty on the Non-Proliferation of Nuclear Weapons (NPT) has accepted IAEA safeguards and inspections for their programs. If they have violated these obligations, they are subject to sanctions.
In short, look at the level of enrichment permitted in any agreement. For a civil nuclear power program, there is no reason whatsoever enrichment above 5% is required. If enrichment to near 20% is permitted, this is tacit acceptance of a state's being a “screwdriver turn” from a nuclear weapon, since the enrichment work required to get from 20% to weapons-grade is so much smaller and easier to conceal than the first two stages.
Acknowledgement: This essay has been informed by E-mail discussions with Robert G. Kennedy, III, who resides in America's Atomic City and knows far more about these matters than this scrivener. Any errors are, of course, my own.
Here is a one hour lecture by Harvard Prof. Matthew Bunn on the design of nuclear weapons:
and a second lecture on the production of nuclear material for weapons, which is the topic of this essay:
|
|
This document is in the public domain.