| 2017 |
January 2017
- Brown, Brandon R. Planck. Oxford: Oxford University Press, 2015. ISBN 978-0-19-021947-5.
-
Theoretical physics is usually a young person's game. Many of the
greatest breakthroughs have been made by researchers in their
twenties, just having mastered existing theories while remaining
intellectually flexible and open to new ideas. Max Planck,
born in 1858, was an exception to this rule. He spent most of his
twenties living with his parents and despairing of finding a
paid position in academia. He was thirty-six when he took on
the project of understanding heat radiation, and forty-two
when he explained it in terms which would launch the quantum
revolution in physics. He was in his fifties when he discovered
the zero-point energy of the vacuum, and remained engaged and active
in science until shortly before his death in 1947 at the age of
89. As theoretical physics editor for the then most
prestigious physics journal in the world,
Annalen der Physik, in 1905 he
approved publication of Einstein's special theory of relativity,
embraced the new ideas from a young outsider with neither a Ph.D. nor
an academic position, extended the theory in his own work in
subsequent years, and was instrumental in persuading Einstein
to come to Berlin, where he became a close friend.
Sometimes the simplest puzzles lead to the most profound of insights.
At the end of the nineteenth century, the radiation emitted by
heated bodies was such a conundrum. All objects emit electromagnetic
radiation due to the thermal motion of their molecules. If an object
is sufficiently hot, such as the filament of an incandescent lamp or
the surface of the Sun, some of the radiation will fall into the
visible range and be perceived as light. Cooler objects emit in
the infrared or lower frequency bands and can be detected by
instruments sensitive to them. The radiation emitted by a hot
object has a characteristic spectrum (the distribution of energy
by frequency), and has a peak which depends only upon the
temperature of the body. One of the simplest cases is that of a
black body,
an ideal object which perfectly absorbs all incident radiation.
Consider an ideal closed oven which loses no heat to the outside.
When heated to a given temperature, its walls will absorb and
re-emit radiation, with the spectrum depending upon its temperature.
But the
equipartition
theorem, a cornerstone of
statistical
mechanics, predicted that the absorption and re-emission of
radiation in the closed oven would result in a ever-increasing
peak frequency and energy, diverging to infinite temperature, the
so-called
ultraviolet
catastrophe. Not only did this violate the law of conservation of
energy, it was an affront to common sense: closed ovens do not explode
like nuclear bombs. And yet the theory which predicted this behaviour,
the
Rayleigh-Jeans
law,
made perfect sense based upon the motion of atoms and molecules,
correctly predicted numerous physical phenomena, and was correct for
thermal radiation at lower temperatures.
At the time Planck took up the problem of thermal radiation,
experimenters in Germany were engaged in measuring the radiation
emitted by hot objects with ever-increasing precision, confirming
the discrepancy between theory and reality, and falsifying several
attempts to explain the measurements. In December 1900, Planck
presented his new theory of black body radiation and what is
now called
Planck's Law
at a conference in Berlin. Written in modern notation, his
formula for the energy emitted by a body of temperature
T at frequency ν is:
This equation not only correctly predicted the results measured in the laboratories, it avoided the ultraviolet catastrophe, as it predicted an absolute cutoff of the highest frequency radiation which could be emitted based upon an object's temperature. This meant that the absorption and re-emission of radiation in the closed oven could never run away to infinity because no energy could be emitted above the limit imposed by the temperature. Fine: the theory explained the measurements. But what did it mean? More than a century later, we're still trying to figure that out. Planck modeled the walls of the oven as a series of resonators, but unlike earlier theories in which each could emit energy at any frequency, he constrained them to produce discrete chunks of energy with a value determined by the frequency emitted. This had the result of imposing a limit on the frequency due to the available energy. While this assumption yielded the correct result, Planck, deeply steeped in the nineteenth century tradition of the continuum, did not initially suggest that energy was actually emitted in discrete packets, considering this aspect of his theory “a purely formal assumption.” Planck's 1900 paper generated little reaction: it was observed to fit the data, but the theory and its implications went over the heads of most physicists. In 1905, in his capacity as editor of Annalen der Physik, he read and approved the publication of Einstein's paper on the photoelectric effect, which explained another physics puzzle by assuming that light was actually emitted in discrete bundles with an energy determined by its frequency. But Planck, whose equation manifested the same property, wasn't ready to go that far. As late as 1913, he wrote of Einstein, “That he might sometimes have overshot the target in his speculations, as for example in his light quantum hypothesis, should not be counted against him too much.” Only in the 1920s did Planck fully accept the implications of his work as embodied in the emerging quantum theory.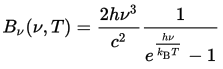
The equation for Planck's Law contained two new fundamental physical constants: Planck's constant (h) and Boltzmann's constant (kB). (Boltzmann's constant was named in memory of Ludwig Boltzmann, the pioneer of statistical mechanics, who committed suicide in 1906. The constant was first introduced by Planck in his theory of thermal radiation.) Planck realised that these new constants, which related the worlds of the very large and very small, together with other physical constants such as the speed of light (c), the gravitational constant (G), and the Coulomb constant (ke), allowed defining a system of units for quantities such as length, mass, time, electric charge, and temperature which were truly fundamental: derived from the properties of the universe we inhabit, and therefore comprehensible to intelligent beings anywhere in the universe. Most systems of measurement are derived from parochial anthropocentric quantities such as the temperature of somebody's armpit or the supposed distance from the north pole to the equator. Planck's natural units have no such dependencies, and when one does physics using them, equations become simpler and more comprehensible. The magnitudes of the Planck units are so far removed from the human scale they're unlikely to find any application outside theoretical physics (imagine speed limit signs expressed in a fraction of the speed of light, or road signs giving distances in Planck lengths of 1.62×10−35 metres), but they reflect the properties of the universe and may indicate the limits of our ability to understand it (for example, it may not be physically meaningful to speak of a distance smaller than the Planck length or an interval shorter than the Planck time [5.39×10−44 seconds]).
Planck's life was long and productive, and he enjoyed robust health (he continued his long hikes in the mountains into his eighties), but was marred by tragedy. His first wife, Marie, died of tuberculosis in 1909. He outlived four of his five children. His son Karl was killed in 1916 in World War I. His two daughters, Grete and Emma, both died in childbirth, in 1917 and 1919. His son and close companion Erwin, who survived capture and imprisonment by the French during World War I, was arrested and executed by the Nazis in 1945 for suspicion of involvement in the Stauffenberg plot to assassinate Hitler. (There is no evidence Erwin was a part of the conspiracy, but he was anti-Nazi and knew some of those involved in the plot.) Planck was repulsed by the Nazis, especially after a private meeting with Hitler in 1933, but continued in his post as the head of the Kaiser Wilhelm Society until 1937. He considered himself a German patriot and never considered emigrating (and doubtless his being 75 years old when Hitler came to power was a consideration). He opposed and resisted the purging of Jews from German scientific institutions and the campaign against “Jewish science”, but when ordered to dismiss non-Aryan members of the Kaiser Wilhelm Society, he complied. When Heisenberg approached him for guidance, he said, “You have come to get my advice on political questions, but I am afraid I can no longer advise you. I see no hope of stopping the catastrophe that is about to engulf all our universities, indeed our whole country. … You simply cannot stop a landslide once it has started.” Planck's house near Berlin was destroyed in an Allied bombing raid in February 1944, and with it a lifetime of his papers, photographs, and correspondence. (He and his second wife Marga had evacuated to Rogätz in 1943 to escape the raids.) As a result, historians have only limited primary sources from which to work, and the present book does an excellent job of recounting the life and science of a man whose work laid part of the foundations of twentieth century science. - Wolfe, Tom. The Kingdom of Speech. New York: Little, Brown, 2016. ISBN 978-0-316-40462-4.
- In this short (192) page book, Tom Wolfe returns to his roots in the “new journalism”, of which he was a pioneer in the 1960s. Here the topic is the theory of evolution; the challenge posed to it by human speech (because no obvious precursor to speech occurs in other animals); attempts, from Darwin to Noam Chomsky to explain this apparent discrepancy and preserve the status of evolution as a “theory of everything”; and the evidence collected by linguist and anthropologist Daniel Everett among the Pirahã people of the Amazon basin in Brazil, which appears to falsify Chomsky's lifetime of work on the origin of human language and the universality of its structure. A second theme is contrasting theorists and intellectuals such as Darwin and Chomsky with “flycatchers” such as Alfred Russel Wallace, Darwin's rival for priority in publishing the theory of evolution, and Daniel Everett, who work in the field—often in remote, unpleasant, and dangerous conditions—to collect the data upon which the grand thinkers erect their castles of hypothesis. Doubtless fearful of the reaction if he suggested the theory of evolution applied to the origin of humans, in his 1859 book On the Origin of Species, Darwin only tiptoed close to the question two pages from the end, writing, “In the distant future, I see open fields for far more important researches. Psychology will be securely based on a new foundation, that of the necessary acquirement of each mental power and capacity of gradation. Light will be thrown on the origin of man and his history.” He needn't have been so cautious: he fooled nobody. The very first review, five days before publication, asked, “If a monkey has become a man—…?”, and the tempest was soon at full force. Darwin's critics, among them Max Müller, German-born professor of languages at Oxford, and Darwin's rival Alfred Wallace, seized upon human characteristics which had no obvious precursors in the animals from which man was supposed to have descended: a hairless body, the capacity for abstract thought, and, Müller's emphasis, speech. As Müller said, “Language is our Rubicon, and no brute will dare cross it.” How could Darwin's theory, which claimed to describe evolution from existing characteristics in ancestor species, explain completely novel properties which animals lacked? Darwin responded with his 1871 The Descent of Man, and Selection in Relation to Sex, which explicitly argued that there were precursors to these supposedly novel human characteristics among animals, and that, for example, human speech was foreshadowed by the mating songs of birds. Sexual selection was suggested as the mechanism by which humans lost their hair, and the roots of a number of human emotions and even religious devotion could be found in the behaviour of dogs. Many found these arguments, presented without any concrete evidence, unpersuasive. The question of the origin of language had become so controversial and toxic that a year later, the Philological Society of London announced it would no longer accept papers on the subject. With the rediscovery of Gregor Mendel's work on genetics and subsequent research in the field, a mechanism which could explain Darwin's evolution was in hand, and the theory became widely accepted, with the few discrepancies set aside (as had the Philological Society) as things we weren't yet ready to figure out. In the years after World War II, the social sciences became afflicted by a case of “physics envy”. The contribution to the war effort by their colleagues in the hard sciences in areas such as radar, atomic energy, and aeronautics had been handsomely rewarded by prestige and funding, while the more squishy sciences remained in a prewar languor along with the departments of Latin, Medieval History, and Drama. Clearly, what was needed was for these fields to adopt a theoretical approach grounded in mathematics which had served so well for chemists, physicists, engineers, and appeared to be working for the new breed of economists. It was into this environment that in the late 1950s a young linguist named Noam Chomsky burst onto the scene. Over its century and a half of history, much of the work of linguistics had been cataloguing and studying the thousands of languages spoken by people around the world, much as entomologists and botanists (or, in the pejorative term of Darwin's age, flycatchers) travelled to distant lands to discover the diversity of nature and try to make sense of how it was all interrelated. In his 1957 book, Syntactic Structures, Chomsky, then just twenty-eight years old and working in the building at MIT where radar had been developed during the war, said all of this tedious and messy field work was unnecessary. Humans had evolved (note, “evolved”) a “language organ”, an actual physical structure within the brain—the “language acquisition device”—which children used to learn and speak the language they heard from their parents. All human languages shared a “universal grammar”, on top of which all the details of specific languages so carefully catalogued in the field were just fluff, like the specific shape and colour of butterflies' wings. Chomsky invented the “Martian linguist” which was to come to feature in his lectures, who he claimed, arriving on Earth, would quickly discover the unity underlying all human languages. No longer need the linguist leave his air conditioned office. As Wolfe writes in chapter 4, “Now, all the new, Higher Things in a linguist's life were to be found indoors, at a desk…looking at learned journals filled with cramped type instead of at a bunch of hambone faces in a cloud of gnats.” Given the alternatives, most linguists opted for the office, and for the prestige that a theory-based approach to their field conferred, and by the 1960s, Chomsky's views had taken over linguistics, with only a few dissenters, at whom Chomsky hurled thunderbolts from his perch on academic Olympus. He transmuted into a general-purpose intellectual, pronouncing on politics, economics, philosophy, history, and whatever occupied his fancy, all with the confidence and certainty he brought to linguistics. Those who dissented he denounced as “frauds”, “liars”, or “charlatans”, including B. F. Skinner, Alan Dershowitz, Jacques Lacan, Elie Wiesel, Christopher Hitchens, and Jacques Derrida. (Well, maybe I agree when it comes to Derrida and Lacan.) In 2002, with two colleagues, he published a new theory claiming that recursion—embedding one thought within another—was a universal property of human language and component of the universal grammar hard-wired into the brain. Since 1977, Daniel Everett had been living with and studying the Pirahã in Brazil, originally as a missionary and later as an academic linguist trained and working in the Chomsky tradition. He was the first person to successfully learn the Pirahã language, and documented it in publications. In 2005 he published a paper in which he concluded that the language, one of the simplest ever described, contained no recursion whatsoever. It also contained neither a past nor future tense, description of relations beyond parents and siblings, gender, numbers, and many additional aspects of other languages. But the absence of recursion falsified Chomsky's theory, which pronounced it a fundamental part of all human languages. Here was a field worker, a flycatcher, braving not only gnats but anacondas, caimans, and just about every tropical disease in the catalogue, knocking the foundation from beneath the great man's fairy castle of theory. Naturally, Chomsky and his acolytes responded with their customary vituperation, (this time, the adjective of choice for Everett was “charlatan”). Just as they were preparing the academic paper which would drive a stake through this nonsense, Everett published Don't Sleep, There Are Snakes, a combined account of his thirty years with the Pirahã and an analysis of their language. The book became a popular hit and won numerous awards. In 2012, Everett followed up with Language: The Cultural Tool, which rejects Chomsky's view of language as an innate and universal human property in favour of the view that it is one among a multitude of artifacts created by human societies as a tool, and necessarily reflects the characteristics of those societies. Chomsky now refuses to discuss Everett's work. In the conclusion, Wolfe comes down on the side of Everett, and argues that the solution to the mystery of how speech evolved is that it didn't evolve at all. Speech is simply a tool which humans used their big brains to invent to help them accomplish their goals, just as they invented bows and arrows, canoes, and microprocessors. It doesn't make any more sense to ask how evolution produced speech than it does to suggest it produced any of those other artifacts not made by animals. He further suggests that the invention of speech proceeded from initial use of sounds as mnemonics for objects and concepts, then progressed to more complex grammatical structure, but I found little evidence in his argument to back the supposition, nor is this a necessary part of viewing speech as an invented artifact. Chomsky's grand theory, like most theories made up without grounding in empirical evidence, is failing both by being falsified on its fundamentals by the work of Everett and others, and also by the failure, despite half a century of progress in neurophysiology, to identify the “language organ” upon which it is based. It's somewhat amusing to see soft science academics rush to Chomsky's defence, when he's arguing that language is biologically determined as opposed to being, as Everett contends, a social construct whose details depend upon the cultural context which created it. A hunter-gatherer society such as the Pirahã living in an environment where food is abundant and little changes over time scales from days to generations, doesn't need a language as complicated as those living in an agricultural society with division of labour, and it shouldn't be a surprise to find their language is more rudimentary. Chomsky assumed that all human languages were universal (able to express any concept), in the sense David Deutsch defined universality in The Beginning of Infinity, but why should every people have a universal language when some cultures get along just fine without universal number systems or alphabets? Doesn't it make a lot more sense to conclude that people settle on a language, like any other tools, which gets the job done? Wolfe then argues that the capacity of speech is the defining characteristic of human beings, and enables all of the other human capabilities and accomplishments which animals lack. I'd consider this not proved. Why isn't the definitive human characteristic the ability to make tools, and language simply one among a multitude of tools humans have invented? This book strikes me as one or two interesting blog posts struggling to escape from a snarknado of Wolfe's 1960s style verbal fireworks, including Bango!, riiippp, OOOF!, and “a regular crotch crusher!”. At age 85, he's still got it, but I wonder whether he, or his editor, questioned whether this style of journalism is as effective when discussing evolutionary biology and linguistics as in mocking sixties radicals, hippies, or pretentious artists and architects. There is some odd typography, as well. Grave accents are used in words like “learnèd”, presumably to indicate it's to be pronounced as two syllables, but then occasionally we get an acute accent instead—what's that supposed to mean? Chapter endnotes are given as superscript letters while source citations are superscript numbers, neither of which are easy to select on a touch-screen Kindle edition. There is no index.